Centrifugation of Stallion Semen
By Jos Mottershead
Why Centrifuge Semen?
There are a number of reasons why one would choose to centrifuge stallion semen other than the obvious reasons such as during preparation prior to freezing.
One of the commonest of reasons is that the sperm concentration of the raw ejaculate is so low that it makes processing of a suitable insemination dose difficult for transported use. Ideally one ships an insemination dose of 1 billion progressively motile sperm (at the time of shipment) which, allowing for an arbitrary 50% die-off rate, provides the desired insemination dose of 500 million progressively motile sperm. In order to achieve a successful shipment of fertile sperm however other parameters must be considered. To achieve a minimum die-off rate sperm should be shipped at a final concentration of between 25 and 50 million per ml; it is also necessary (if using a NFDMS such as Kenney extender) to extend the raw semen at a minimum rate of one part of semen to three parts of extender (1:4 or greater may be preferable); and finally, it is desirable that the total volume of the insemination dose be no more than approximately 80 ml (and 50 ml is more desirable yet). All of these parameters may not be obtainable if the initial sperm concentration of the ejaculate is too low – typically if below 100 million sperm/ml at the time of collection.
Another common reason for centrifugation of semen is to remove seminal plasma which is harmful to sperm over an extended period of exposure. It is one of nature’s little jokes that all equine seminal plasma is toxic to sperm over an extended period, however with some stallions this negative effect is far greater than others, and with those animals it becomes essential to not just dilute semen with extender, but to actually remove the majority of the seminal plasma prior to extender addition. With many so-called “poor longevity” stallions, this will result in semen that may be successfully stored or transported, whereas without centrifugation the semen must be used almost immediately.
Interestingly, in Europe, centrifugation of semen prior to cooled shipment is extremely common, to the point of being unusual if not done. On the contrary, in North America, centrifugation prior to cooled shipment is rarely done and if it is done it is usually as a result of a low initial concentration or seminal plasma toxicity. The argument may be made in Europe that the centrifugation prolongs the longevity of the sperm, which while true is somewhat puzzling, as in many instances owing to the shorter distances, the semen even though being transported is often in the mare the same day there. In North America, shipped semen is routinely transported by overnight and the majority is inseminated at least 24 hours after collection, with a second dose being sent (again, another rarity in Europe) which is inseminated 24 hours after that. It seems therefore that in most cases a routine removal of seminal plasma is unnecessary. Indeed, it could easily be argued that the beneficial qualities of seminal plasma relative to uterine quiescence are of value, so that removal in most cases is not only not necessary, but potentially detrimental. The overall effect of routine removal or non-removal however is the telling factor, and as both practices result in acceptable pregnancy rates, the routine removal can perhaps simply be regarded as an unnecessary added inconvenience in most cases.
Centrifuge Size

 While the slightly more advanced breeder wants to avoid having to purchase too much additional equipment, dual use of a centrifuge for semen and serum (for progesterone or other blood-hormone assays) may be a little tricky, although technically possible. For centrifugation of stallion semen one will ideally have a centrifuge that will hold 50 ml tubes, whereas for serum one wants one that holds 15 ml tubes. How to get around this problem will depend a little on what one is planning on using the semen for – i.e. freezing it, or just super-concentrating it for cooled or immediate use. If planning on freezing, there really is no option but to get a centrifuge that accepts 50 ml tubes, as it is essential that there is a minimum of handling done in the shortest time frame, typically at lower speeds. If one is going to use the centrifuge for super-concentrating fresh semen for cooled or immediate use, one can get away with using a centrifuge that accepts a 15 ml tube. There is a possibly convenient alternative, which would be to have two different rotors for the same centrifuge, but one would have to go with a larger size centrifuge in order for it to accept the 50 ml tubes, and the larger machines do not spin as fast as the smaller, which would means that one would probably have to spin the blood for longer – not a big issue really, just a little inconvenient.
While the slightly more advanced breeder wants to avoid having to purchase too much additional equipment, dual use of a centrifuge for semen and serum (for progesterone or other blood-hormone assays) may be a little tricky, although technically possible. For centrifugation of stallion semen one will ideally have a centrifuge that will hold 50 ml tubes, whereas for serum one wants one that holds 15 ml tubes. How to get around this problem will depend a little on what one is planning on using the semen for – i.e. freezing it, or just super-concentrating it for cooled or immediate use. If planning on freezing, there really is no option but to get a centrifuge that accepts 50 ml tubes, as it is essential that there is a minimum of handling done in the shortest time frame, typically at lower speeds. If one is going to use the centrifuge for super-concentrating fresh semen for cooled or immediate use, one can get away with using a centrifuge that accepts a 15 ml tube. There is a possibly convenient alternative, which would be to have two different rotors for the same centrifuge, but one would have to go with a larger size centrifuge in order for it to accept the 50 ml tubes, and the larger machines do not spin as fast as the smaller, which would means that one would probably have to spin the blood for longer – not a big issue really, just a little inconvenient.
The Process
With a Cushion
If a centrifugation cushion is not being used, then the speed and duration of centrifuging will vary a little from stallion to stallion and depending upon the end use intended for the resulting sperm pellet. For simplicity, the use of a centrifugation cushion is recommended. There are several such products available commercially, such as “Maxifreeze” (IMV) or “RedCushion” (Botupharma), of which a small quantity (½ to 1ml) is layered beneath the extended semen in the centrifugation tube. The cushion is a dense media which remains at the bottom of the tube, and the sperm layer is formed immediately above it. The advantage of the use of a cushion is that one can uniformly centrifuge most stallion’s semen with successful sperm recovery rates (90-95%) at 1,000 G for 20 minutes. Once completed, the supernatant (the plasma/extender portion in the upper section of the tube) can be suctioned off, then the cushion (if desired) removed from underneath the sperm layer. It is generally recommended that 5-10% seminal plasma be left to assist with a reduction in the mare’s inflammatory response and improve sperm motility, however this does depend upon the level of toxicity towards the stallion’s sperm if that is the problem being addressed.
Without a Cushion
If one is using a 15 ml centrifuge, and the sperm pellet is not to be frozen, then two to three minutes at a mid-point setting should be sufficient to concentrate the upper portion into the lower portion. There will still be some sperm left in the supernatant, but the majority of the sperm will be at, or towards, the bottom in a loose pellet. Removal of the upper 10 cc of the supernatant should give a sufficient yield in the remaining 5 ml. One should however check the supernatant, and if there are still a lot of sperm left, one may wish to increase the duration of the spin. Re-counting the sperm concentration to determine recovery rate will be necessary, and this becomes a chore if an extender is used as photometric counting devices (Densimeter, Spermacue, QuickCheck etc.) cannot be used owing to the opaque nature of the extender. If the short-duration spin is being used, one can try to centrifuge without extender to see what the impact is on the sperm, however if seminal plasma toxicity is a concern, clearly this method will not produce optimal results.
If a 50 ml tube is being used, the same protocol can be used, although results may be less predictable. Counting will again be required to determine an average recovery rate (which will likely be around 75%). Essentially, the starting point for producing a sperm pellet would be 400 G for 6 minutes (“G-Force”, “Relative Centrifugal Force” and “RCF” are all the same thing). Maximal sperm recovery is obviously desired, without damaging the sperm. If the sperm pellet is hard and cannot easily be resuspended, then either too long a duration or too hard a spin was performed and adjustments will be required. A soft, easily resuspended sperm pellet is desired. With experimentation, one will find the optimum time and settings for a particular stallion. Some stallion’s semen will tolerate being spun harder for a shorter period, some require a lower G for a longer period. I have had some that when spun at that starting point, give me a nervous breakdown when I resuspend and look at it under a microscope, as there’s only 5% progressive motility! Stallion variability has the potential to be significant, so the value of using a centrifugation cushion quickly becomes apparent.
How Does the Setting on the Centrifuge Equate to Relative Centrifugal Force (rcf or “g-force”)?
The formula is:
Relative Centrifugal Force (RCF or G-value) = (11.18) x ( r ) x (n x n) x (10-6)
Where: r = Centrifugal radius in cm (distance between rotational center and sample center); and n = revolutions per minute
So, for example:
If the radius were 10 cm, and the revolutions per minute were 1900, the RCF (“G force”) would be:
(11.18) x (10) x (1900 x 1900) x (10-6)
or:
(11.18) x (10) (3,610,000) x (10-6)
or:
(403,598,000) x (10-6)
or:
about 403.6 G (rounded up to 404).
To obtain an accurate determination of RCF, one must have the centrifuge rotation speed calibrated. This can be achieved with the built in or hand-held RPM tool and performing the above calculations. If there isn’t a method of checking settings available, then it is possible (although not preferable) to “play it by ear”. Spinning in a 50 ml centrifuge at 400 G is not really fast. It is under the halfway setting on the old IEC “Clinical” model centrifuge (the CL2 model provides a setting to give a constant RPM). Note that if a centrifuge is being used which does not provide a constant RPM setting, voltage fluctuations may result in different speeds in different locations.
Single Layer Centrifugation
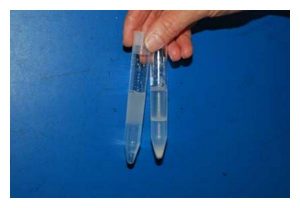
The tube on the left shows the SLC-4.5 prior to centrifugation, with 4.5 mL extended boar semen on top of 4.0 mL Androcoll-P. The tube on the right shows the preparation after centrifugation with the sperm pellet clearly visible in the bottom of the tube. Note the white line marking the interface between semen and colloid after centrifugation. This consists of spermatozoa that have not been able to pass into the colloid because of poor motility, abnormal morphology, or damaged chromatin.
Source: Morrell JM, van Wienen M, Wallgren M. 2011. Single Layer Centrifugation Can Be Scaled-Up Further to Process up to 150mL Semen. ISRN Vet Sci:2011:183412
https://pubmed.ncbi.nlm.nih.gov/23738111/
Use of a colloidal (“Androcoll-E” or “EquiPure”) for centrifugation of stallion semen has been shown to result in improved sperm morphological percentages and motility post-processing as well as reducing bacterial or viral load in the ejaculate (Morrell JM, Kumaresan A, Johannisson A. 2017. Practical implications of sperm selection techniques for improving reproduction. Anim Reprod, 14:572-580). One can also use the technique to improve received semen prior to insemination, with both fresh-cooled and frozen semen. Extended semen is placed over a layer of the centrifugation medium (or alternatively, the extended semen can be placed in the centrifuge tube first, and the medium layered underneath the semen). While 15-ml tubes are widely used, with the comparatively high volume seen with stallion ejaculates, the use of a 50-ml centrifuge tube is more practical, with semen centrifuged in both cases at 300 g for 20 minutes (Morrell JM, Johannisson A, Dalin A-M, Rodriguez-Martinez H. 2009. Single-layer centrifugation with Androcoll-E can be scaled up to allow large volumes of stallion ejaculate to be processed easily. Theriogenology 72;6:879-884). After centrifugation, the sperm layer found in the bottom of the conical centrifuge tube will have improved morphological and motility parameters with improved fertility. While this can be clearly beneficial for stallions with related problems, research has also suggested that improved fertility is seen with even “normal” ejaculates (Morrell JM, Richter J, Martinsson G, Stuhtmann G, Hoogewijs M, Roels K, Dalin AM. 2014. Pregnancy rates after artificial insemination with cooled stallion spermatozoa either with or without single layer centrifugation. Theriogenology 82(8):1102-5). This should however be balanced against increased costs and how much improvement is seen in the individual animal to determine if it is practical for the specific situation.
The following video courtesy of Botupharma demonstrates use of the single-layer centrifugation medium to improve sperm motility with received semen. Techniques and equipment may vary slightly between laboratories and technicians, but this demonstrates the basic technique:
Using Centrifugation to Remove Gel Fraction
Some people have suggested using centrifugation to remove the gel fraction of an ejaculate, if present. I have had limited success with this method, and have found it to be a tricky process, with success rates that seem to vary from stallion to stallion. If one can get the centrifugation rate set to allow a layering effect, with the sperm pellet at the bottom, and then the layer of gel, I have found that the gel layer can be removed by sucking it off with a shortened insemination pipette on a syringe. Attempting to remove it with a syringe and needle doesn’t work as it is too dense for the needle. I have even tried using an IVF catheter, which has a larger bore than a needle (but smaller than an insemination pipette), but still without success. The pipette however allows the “picking up” of the gel portion – almost in the same manner as separating an egg white out from a yolk. I did find though, that some stallions had gel that would centrifuge into the sperm pellet, or others required such a hard centrifugation rate to separate it that the sperm were damaged. I had one stallion that would ejaculate about 50 ml gel free and 200 ml of gel, and with him we would not centrifuge, but simply triple or quadruple filter the semen until we had removed all the gel fraction, using milk filters – the filters from ARS for the Colorado AV were too fine and simply clogged. Fractionation of the ejaculate at the time of collection to capture only the first three ejaculatory (sperm-rich) spurts may be an easier solution with a stallion with high gel volume.
Don’t…
A final “Don’t”…:
- Don’t use the rotor brake on the centrifuge if it has one when stopping it after centrifugation of stallion semen. Allow it to slow down by itself, otherwise sperm damage may occur.
© 1999, 2007, 2015, 2020, 2022 Equine-Reproduction.com, LLC
Use of article permitted only upon receipt of required permission and with necessary accreditation.
Please contact us for further details of article use requirements.
Other conditions may apply.



